Lead Iodide Reaction Class 10 . A yellow precipitate of lead iodide. Web activity 1.2 ncert science class 10 chemical reactions and equations. It can also be called a precipitate reaction as an insoluble solid substance[precipitate] formed during the activity. Web precipitation reaction between potassium iodide and lead. The pbi 2 is generally synthesized by the process of a precipitation reaction between the potassium iodide i.e. Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Web activity 1.2 from class 10 science ncert book chapter 1 effectively demonstrates a double displacement. Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you.
from edurev.in
Web activity 1.2 ncert science class 10 chemical reactions and equations. A yellow precipitate of lead iodide. Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Web activity 1.2 from class 10 science ncert book chapter 1 effectively demonstrates a double displacement. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. It can also be called a precipitate reaction as an insoluble solid substance[precipitate] formed during the activity. The pbi 2 is generally synthesized by the process of a precipitation reaction between the potassium iodide i.e. Web precipitation reaction between potassium iodide and lead.
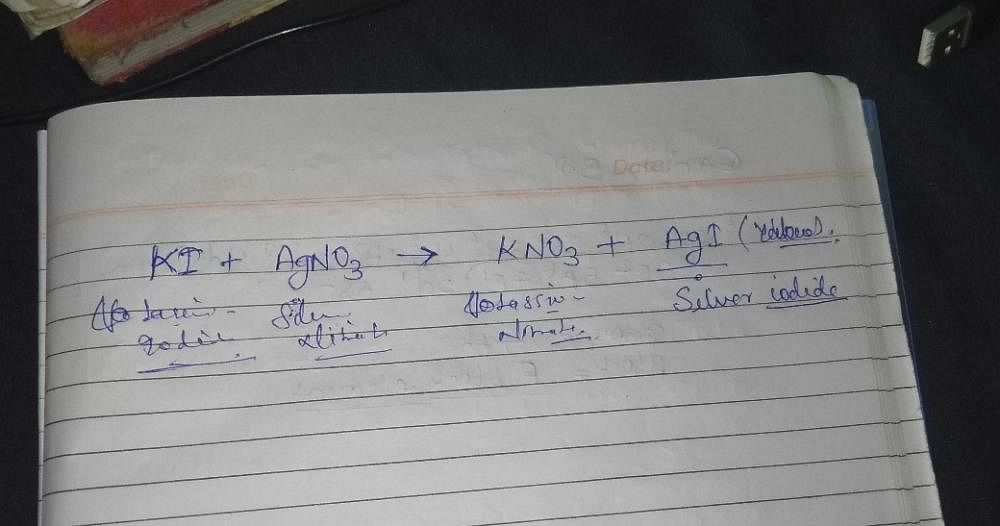
What is observed when a solution of potassium iodide is added to silver
Lead Iodide Reaction Class 10 The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. A yellow precipitate of lead iodide. Web activity 1.2 ncert science class 10 chemical reactions and equations. The pbi 2 is generally synthesized by the process of a precipitation reaction between the potassium iodide i.e. Web precipitation reaction between potassium iodide and lead. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. Web activity 1.2 from class 10 science ncert book chapter 1 effectively demonstrates a double displacement. It can also be called a precipitate reaction as an insoluble solid substance[precipitate] formed during the activity. Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you.
From www.chegg.com
Solved Lead (II) nitrate reacts with sodium iodide to Lead Iodide Reaction Class 10 The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. The pbi 2 is generally synthesized by the process of a precipitation reaction between the potassium iodide i.e. Web. Lead Iodide Reaction Class 10.
From www.flickr.com
Lead Iodide Pittsburgh Carnegie Science and Trib Total Med… Flickr Lead Iodide Reaction Class 10 Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. The pbi 2 is generally synthesized by the process of a precipitation reaction between the potassium iodide i.e. Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. A yellow precipitate of lead iodide. Web activity 1.2 from. Lead Iodide Reaction Class 10.
From www.youtube.com
Lead Nitrate and Potassium Iodide Reaction ChemistryBimistry Labs Lead Iodide Reaction Class 10 Web precipitation reaction between potassium iodide and lead. It can also be called a precipitate reaction as an insoluble solid substance[precipitate] formed during the activity. Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. Web activity 1.2. Lead Iodide Reaction Class 10.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead iodide Fundamental Lead Iodide Reaction Class 10 A yellow precipitate of lead iodide. Web activity 1.2 ncert science class 10 chemical reactions and equations. Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. It can also be called a precipitate reaction as an insoluble solid substance[precipitate] formed during the activity. Web this activity shows a double displacement reaction between. Lead Iodide Reaction Class 10.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead iodide Fundamental Lead Iodide Reaction Class 10 Web activity 1.2 from class 10 science ncert book chapter 1 effectively demonstrates a double displacement. Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. The pbi 2 is generally synthesized by the process of a precipitation reaction between the potassium iodide i.e. It can also be called a precipitate reaction as. Lead Iodide Reaction Class 10.
From www.pw.live
Double Displacement and Reactions Physics Wallah Lead Iodide Reaction Class 10 Web activity 1.2 ncert science class 10 chemical reactions and equations. Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. The pbi 2 is generally synthesized by the process of a precipitation reaction between the potassium iodide i.e. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that. Lead Iodide Reaction Class 10.
From www.teachoo.com
In the double displacement reaction b/w aqueo (MCQ) Class 10 Science Lead Iodide Reaction Class 10 Web activity 1.2 from class 10 science ncert book chapter 1 effectively demonstrates a double displacement. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Web precipitation reaction between potassium iodide and lead. Web lead nitrate reacts with potassium iodide to produce a. Lead Iodide Reaction Class 10.
From www.toppr.com
\"Reaction of potassium iodide solution with lead nitrate solution Lead Iodide Reaction Class 10 Web precipitation reaction between potassium iodide and lead. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. A yellow precipitate of lead iodide. Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. Web activity 1.2 from class. Lead Iodide Reaction Class 10.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead iodide Fundamental Lead Iodide Reaction Class 10 The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Web activity 1.2 ncert science class 10 chemical reactions and equations. Web precipitation reaction between potassium iodide and lead. The pbi 2 is generally synthesized by the process of a precipitation reaction between the. Lead Iodide Reaction Class 10.
From www.youtube.com
(4/1) Reaction between Lead nitrate& potassium iodide10thNcert Lead Iodide Reaction Class 10 The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. The pbi 2 is generally synthesized by the process of a precipitation reaction between the potassium iodide i.e. Web precipitation reaction between potassium iodide and lead. Web activity 1.2 ncert science class 10 chemical. Lead Iodide Reaction Class 10.
From webapi.bu.edu
Lead iodide formula. Lead Iodide Structure, Preparations and Lead Iodide Reaction Class 10 Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Web activity 1.2 ncert science class 10 chemical reactions and equations. Web activity 1.2 from class 10 science ncert book chapter 1 effectively demonstrates a double displacement. The. Lead Iodide Reaction Class 10.
From www.alamy.com
solutions of lead nitrate and potassium iodide will react together to Lead Iodide Reaction Class 10 Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. It can also be called a precipitate reaction as an insoluble solid substance[precipitate] formed during the activity. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. A yellow. Lead Iodide Reaction Class 10.
From brainly.in
What happen when lead nitrate reacts with potassium iodide solutions Lead Iodide Reaction Class 10 Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. It can also be called a precipitate reaction as an insoluble solid substance[precipitate] formed during the activity. Web precipitation reaction between potassium iodide and lead. Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. The reaction, known. Lead Iodide Reaction Class 10.
From www.youtube.com
Experiment on Lead nitrate and Potassium iodide I Class 10 Chemistry Lead Iodide Reaction Class 10 Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. Web activity 1.2 from class 10 science ncert book chapter 1 effectively demonstrates a double displacement. Web precipitation reaction between potassium iodide and lead. Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Web activity 1.2 ncert. Lead Iodide Reaction Class 10.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Lead Iodide Reaction Class 10 Web activity 1.2 from class 10 science ncert book chapter 1 effectively demonstrates a double displacement. Web precipitation reaction between potassium iodide and lead. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. It can also be called a precipitate reaction as an. Lead Iodide Reaction Class 10.
From www.numerade.com
SOLVED Lead iodide (PbI2) dissociates in solution according to the Lead Iodide Reaction Class 10 Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. A yellow precipitate of lead iodide. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Web activity 1.2 from class 10 science ncert book chapter 1 effectively demonstrates. Lead Iodide Reaction Class 10.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Lead Iodide Reaction Class 10 Web precipitation reaction between potassium iodide and lead. It can also be called a precipitate reaction as an insoluble solid substance[precipitate] formed during the activity. Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. Web activity 1.2 ncert science class 10 chemical reactions and equations. The reaction, known as the “golden rain” experiment, produces. Lead Iodide Reaction Class 10.
From www.compoundchem.com
Chemical Reactions Lead Iodide & ‘Golden Rain’ Compound Interest Lead Iodide Reaction Class 10 It can also be called a precipitate reaction as an insoluble solid substance[precipitate] formed during the activity. Web this activity shows a double displacement reaction between lead nitrate and potassium iodide solution. Web lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal. Lead Iodide Reaction Class 10.